Essential Chemistry for Safe Aromatherapy

Sue Clarke Essential Chemistry for Safe Aromatherapy
with foreword by Robert Tisserand
Reviewed by Tony Burfield
pub. Harcourt Health Sciences Ltd (2002) ISBN: 0-443-06485-7
Please note this review is only of the original version.
The need for a good chemistry primer in aromatherapy teaching is self-apparent. Up to now many tutors might have used the corrected 1997 version of the standard work: The Chemistry of Essential Oils by David Williams, which covers the subject but really leans as bit more in the perfumery direction. The question is therefore – is this publication a superior choice for aromatherapy purposes, or is it a better recourse to buy a standard college book on organic chemistry?
My father used to quote Alexander Pope:
“A little learning is a dangerous thing, Drink deep or taste not the Pierian spring”.
….and in my opinion it’s a pity the author and the assembled listed helpers of Essential Chemistry for Safe Aromatherapy did not heed this advice. The 231-page book is certainly nicely set out and presented, but the content shows lack of a deep understanding of the subject matter, resulting in quite serious omissions and ill-advised statements in places. It is a great surprise to me that Robert Tisserand agreed to write the forward to this book.
In general, there is an almost complete lack of biochemistry within the pages - no description of the biogenesis of essential oils is given, and we are led to believe the ‘old howler’ that terpenes are built up from isoprene units, an incorrect belief which is repeated a number of times in the text. This historical (1887) way of looking at terpene structures may still be useful (see below), but bears no relation to our modern understandings of biochemical pathways. There’s also a curious reluctance to use the word “aromatic” for certain non-terpinic essential oil constituents as well – as on page 58 “eugenol is not actually derived from a terpene molecule” or page 70 “benzyl acetate is not a terpene derivative”. Reading the book in detail, there is a feeling that the author feels that only terpenoids really “count” in essential oils, whereas in reality there are many other constituents of essential oils, including phenyl propanoids from the Shikimic acid pathway, and their biotransformation products, and other compounds from the metabolism of fatty acids and amino acids. As well as these important groups of compounds, a large number of other types of chemical components also occur, including nitrogen & sulphur compounds. These hardly get a mention.
The lack of biochemical comprehension continues with almost no reference to the isomers of aromatics and terpenes whatever within the book. Differences in metabolism and biodegradation, and the different therapeutic effects of different isomers of the same compound are not mentioned, only the fact that they appear to smell different! Chirality is pretty central to how essential oils help weave their physiological and psychophysiological effects in many instances, but doesn’t seem to be explored at all by the author. Further when describing compounds that occur in essential oils, no mention is made of optical purity of chiral compounds contained therein (often a key factor in deciding whether an oil is authentic or not), or the fact that certain isomers do not tend to occur in nature. Really this dumbing down of the chemistry of natural products, by ignoring all aspects of isomer chemistry, seriously detracts from the usefulness of this book.
Further, an older part of aromatherapy dogma (the functional group theory) is trotted out throughout the book. This attempted to explain the therapeutic properties of individual essential oils via some of the functional groups which occur in its major oil constituents e.g. “ketones are calming and sedative”; “lactones are uplifting yet sedative.” Surely only aromatherapy fundamentalists can now cling to these simplistic views?
In more detail, just some of the mistakes, omissions and incorrect assumptions are set down below as follows:
p38 “Aldehydic smelling oils are due to compounds called aldehydes.”
Comment: In fact the term “aldehydic smelling” is borrowed from mainstream perfumery to describe lower straight chain fatty aldehydes, such as aldehyde C8 (1-octanal) or aldehyde C10 (1-decanal). We don’t have any oils in common use in aromatherapy with appreciable amounts of these components - possibly coriander leaf oil (rarely used) or terpeneless citrus oils (expensive, although Gattefossé used certain terpeneless oils) – but in any case, if we did, they wouldn’t be skin safe. We do have aromatic aldehyde containing oils (cinnamon bark) and acycyclic monoterpene aldehyde containing oils (litsea cubeba oil, lemongrass oil) but you wouldn’t call these “aldehydic” smelling.
p40 “It’s the oxygenated constituents which have significant impact and along with sesquiterpenes, determine and characterise the odours of almost all essential oils.”
Comment: The author has a very worrying tendency to make sweeping statements which are often absolutely & entirely incorrect. True, oxygenated compounds generally have a odour value than monoterpene hydrocarbons, which have a low impact. Also true that in many instances oxygenated compounds are important in characterising odour profiles of essential oils e.g. (+)-carvone in caraway seed oil. I don’t agree with the author on her remarks about sequiterpenes. If anything sesquiterpene hydrocarbons are quite weakly odoured, although sesquiterpene alcohols can be susbstantive. But the statement concerning the contribution of oxygenates to determine and characterise the odour profiles of “almost all essential oils” can’t be susbtantiated. For example the character component of grapefruit oil is a thiol. For buchu oil, a terpinic sulpur compound. For garlic oil: alkyl sulphides. The same for onion and leek oils. Horseradish and mustard oil character components are isocyanates….for Helichrysum italicum the curry note is provided by two sesquiterpene hydrocarbons. Nitrogen compounds also make contributions in the form of pyrazines for example in coriander and galbanum, and in oximes, nitriles and nitro-compounds variously in the head space and essential oils of flowers ….and so on....
p43 “The isoprene unit acts as a monomer or single unit that builds up in repeating units to make the groups of terpenes found in essential oils”
Comment: Wrong! Sure, Wallach in 1887 did propose the “isoprene rule” where monoterpenoids were hypothetically constructed by linkage of isoprene units (in head to tail form). But following Ruzicka’s “biogenic isoprene rule” (1959) it is now assumed that each member of a terpene sub-group - monoterpenes, sesquiterpenes etc. - is derived from a single parent compound, by enzymic mediation. For monoterpenoids like myrcene it is via the parent compound geranyl pyrophosphate (GPP). For sequiterpenoids it is via farnesyl pryrophoshate (FPP). These parent compounds compounds themselves arise from mevalonic acid, which is the precursor of all terpenoids: the pathway is named therefore the Mevalonic Pathway.
p44 “d-limonene found in essential citrus oils, pine leaves and peppermint.”
Comment: Incorrect! Laevo-limonene predominates in conifer and Mentha oils.
p44 Two isoprene molecules diagrammatically shown to form myrcene molecule…
Comment: See above remarks on the isoprene rule.
p44 “Different acyclic monoterpenes are made up of two isoprene units”
Comment:Only hypothetically: see above remarks on the isoprene rule.
p44 & p45. The dumbing down of the text, presumably to make it easy for students to read, imparts almost no information whatsoever and is almost terminally boring:
“Ocimene – found in essential oil of basil”
“Terpinene is found in the essential oils of tea tree and juniper.”
“Pinenes found in essential oils of juniper, pine and cajeput”.
Wouldn’t is be more interesting to say something like:
a-Pinene. (2,6,6-trimethylbicyclo(3,1,1)-2-heptene). The occurrence of this substance is almost ubiquitous in essential oils, and has a pine-like odour with poor tenacity.

- The (+)- isomer occurs in oil of Pinus palustris oil up to 65% and in Eucalyptus oils including E. globulus. Industrially, (+)- pinene has been available ex-E. globulus.
- The (-)-isomer occurs in Pinus caribaea up to 70%, but is more usually obtained industrially from gum turpentine.…
p48. “Caryophyllene”.
Comment: Presumably the author means (-)-b-caryophyllene?
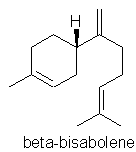
p48. “Bisabolene” – isomer not stated.
Comment: Text says: “found in myrrh oil and German chamomile”, but the formula shown appears to be incorrect for the common a-, b- & g- forms. Here is the correct formula for b-bisabolene:
p50. “Only a few sequiterpenes and their derivatives are volatile – notably the azulenes such as chamazulene, bisabolol and farnescene”.
Comment: Complete rubbish! Sesquiterpenes are common components of very many essential oils and if they were not steam volatile they would not appear in essential oils at all. Note that azulenes are not sesquiterpenes as Sue Clarke maintains, but aromatic hydrocarbons derived from bicyclo[5.3.0]decapentaene.
p52 “Polyterpenes…are obviously outside the scope of aromatherapy.”
Comment: If only! As oils age, the contained terpenes dimerise, trimerise….and polymerise. In spite of the common miscomprehension, a GC trace doesn’t tell you everything about an oil’s composition, let alone about terpene polymers, but HPLC can be employed to reveal this aspect of the aging of all stored essential oils.
p53 “Alcohols are usually hazard-free and non skin-irritating.”
Comment: In fact both the monoterpene alcohols linalool and citronellol are considered to be allergens under current EU legislation. Under the 38th amendment to the IFRA standard “linalool and products known to be rich in linalool, such as bois de rose, coriander or ho wood oils, should only be used when the level of peroxides is kept to the lowest practical value. The addition of 0.1% BHT or a-tocopherol has shown great efficiency. The maximum peroxide level for products in use should be 20mmol/l.”
p60. Phenolic ethers…
Comment: – the toxicity of anethole and methyl chavicol are discussed in the text, although since the book was published, the perceived risks associated with their aromatherapeutic use, arguably, appears to have diminished slightly. Safrole is however described as having been used medicinally as a counterirritant and for parasitic infections (sure, and we used to put copper sulphate in canned peas once upon-a-time, to colour them green….!). Safrole is also described as a component of camphor and sassafras oils but the author fails to report either its carcinogenic potential, its restriction in food and drink legislation, or and its global restriction under controlled substances measures (which high safrole containing oils cannot be traded except under Home Office licence in the UK, or used in aromatherapy). Please note this section does not comply with current national legal requirements and fails to inform of potential health risks to aromatherapists and their clients.
p64 & p115. “Quenchers” (the presence of a distinct chemical substance, also used as an ingredient of a fragrance compound, that will inhibit the sensitising capacity of another substance) are mentioned with regard to citral contaning oils and cinnamic aldehyde.
Comment: Please note that many in the aroma industry doubted the existence of a quenching effect at all when it was first muted, and sure enough its’ scientific credibility has since been queried in the literature; IFRA have now stated that the quenching phenomenon cannot be verified at least with respect to cinnamic aldehyde (Notification of IFRA Standards No. 4 – 38th Amendment, April 6th 2004). So it is a shame to see dubious science passed on in an influential textbook. Note also, that following the findings of Steltenkamp et al. (1980), concentrations of citral over 1% are considered capable of producing skin reactions. The author warns that citral is a very powerful irritant but does not give same emphasis to cinnamic aldehyde in this section (to be fair it is mentioned elsewhere on p191 under irritation). As anyone who works with bulk oils (cinnamon bark, cassia) will tell you, cinnamic aldehyde containing oils are far worse to handle, frequently causing reddening and irritation of the face neck and arms of workers and other areas, if merely exposed to the oil vapour! Under the 38th Amendment to the IFRA Standards, the new limits for cinnamic aldehyde in fragrances for both leave on and rinse-off products is 0.05% (0.5% for non-skin contact products). Limonene and cinnamic aldehyde are considered allergens under the 7th Amendment of the EU Cosmetics Act, and citral and cinnamic aldehyde are additionally considered to be sensitisers and irritants, so these pages contain the most irresponcible advice, putting any potential recipients of these unholy mixes described in the text in a very risky position as far as unpredictable dermal consequences are concerned.
The final mockery comes with the statement on p64 that therapeutically, aldehydes are considered anti-inflammatory. That’s a piece of aromatherapy dogma that has no place in modern teaching. Or indeed in any modern text-book!
p72. Lactones . “Only found in expressed oils and some absolutes”.
Comment: – Just plain wrong! Coumarin itself is found in concentrations of up to 11% in oil of cassia for example. “Umbelliferone: found in many plants” – well, yes but not actually in any steam distilled oils!
p74 Coumarins found in essential oils…. Heniarin
Comment: I am willing to be proved wrong but I think this substance is only present in steam-distilled essential oils at maximum concentrations of a few ppm.
p74 “Lactones found in essential oils: achilline, costuslactone (? - Costus oil is in fact 50% dehydrocostus lactone & costunolide), alantrolactone (sic: should be alantolatone), epinepetalactone, nepetalactone.”
Comment: Although coumarins are stated to be skin sensitive and phototoxic, no mention is made of the connection between sesquiterpene lactones and allergic effects (sesquiterpenes not identified as a group, but examples of sequiterpene lactones are bundled in with the lactones section). The fact is that several species of the Asteraceae which contain sesquiterpene lactones, cause allergy, because they contain a a-methylene-g-lactone grouping, which link to dermal proteins and act as haptens. Oils in this category include Chamomiles (Roman & German), Elecampne oil (Inula helenium), Arnica, Feverfew, Yarrow, Cosmos, Costus (Saussurea lappa). Apart from skin sensitivity issues, Costus is a threatened species under CITES.
p94. “All reputable suppliers will be able to provide you with a GC chromatogram, to give you an indication of the major components of an essential oil”.
Comment: Presumably we would rather know the percentages of the character components and the indicators of authenticity for that oil - these are often the minor, not major, components. Unfortunately oil customers rarely get this information – just an un-interpreted GC graph they can’t understand.
p96-7 Geranium chromatogram.
Comment: Rather interesting to compare with the chromatogram trace run at higher sensitivity in the David Williams book mentioned above, which also boasts a better description of the contributors to the odour characteristics…
p103 “Optical rotation … Santalum album –15° to –20° (no temperature indicated)…. any deviations from this range is a good indication that the oil is not pure.”
Comment: ISO 3518 (2002) indicates an O.R. of -21° to -12° @ 20°C. From my experience I think the latter is a more reliable range than that quoted above.
p104 “If a sample of rosewood (oil) has a S.G. of less than 0.872 (no temperature indicated) it could be due to the addition of extra linalool”.
Comment: The S.G. is merely an indication of authenticity, but not necessarily an exacting criteria, given that Rosewood oil can easily naturally contain 96% of linalool isomers anyway. A better basis to decide whether the sample is authentic is surely from the consideration of the concentration of minor components (eremophilene, hotrienols etc) and an expert assessment of the odour (?).
In any case there is a strong ethical argument that Rosewood oil (Aniba rosaedora) should not be used in aromatherapy, as it is a threatened species.
p114 “Linalool has an almost identical molecular formula (to methyl chavicol) but is a long chain rather than a benzene ring and is considered much safer”
Comment: Possibly gets the prize for the most incomprehensible sentence I have ever seen written in a textbook. I had thought the author was trying to say that the empirical formulae of linalool (C10H18O) and methyl chavicol C10H12O) are identical, but this clearly isn’t the case. The molecular formulae for the two isomers of linalool (acyclic monoterpene alcohols) against methyl chavicol (a phenolic ether) are very, very different and are shown below:
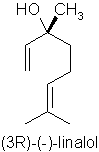

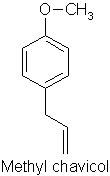
Err…. so its an enigma! Rather like the obscuration of the true meaning of “China Pig” on Captain Beefheart’s Troutmask Replica, perhaps we will never know what was originally intended…!! In any case, as mentioned above, linalool is considered to cause allergic reactions from hydroperoxide formation.
p118. Another hoary old chestnut is presented about rectified Eucalyptus oil. This has come up so many times its amazing that the entire aromatherapy world does not yet fully appreciate the situation. The plain fact is that crude Eucalyptus golubulus oils are normally rectified over 1-2% sodium hydroxide to polymerise and thus effectively remove harmful lower aliphatic aldehydes, such as isovaleric aldehyde, which are not only unpleasantly odoured, and have undesirable toxicological effects, but which can cause uncontrolled fits of coughing in some subjects.
p121 “Bergamot is an essential oil with a potentially harmful constituent and an example of a situation where an adulterated oil can be acceptable”.
Comment: Scarcely believable! - condoning adulteration is not professionally acceptable behaviour in any aromatherapy code of practice that I’m aware of! Goodness knows what message this conveys to students. Those with even a passing knowledge of essential oils, use distilled bergamot oil in skin-sensitive applications, which doesn’t have harmful furanocoumarins. And that’s precisely what the majority of professional aromatherapists use….
p124. Many of species names spelt wrongly: Matricariarecutita, Ormenis mixta, Ormenis multicaulis are the correct spellings. Chamazulene is wrongly described as sesquiterpene.
p127. In spite of dismissing British Pharmacopoeia standards as being “too broadly based for aromatherapy” (?) on p118, the author then presents data which fails to distinguish analytically the difference between Eucalyptus globulus and cineol-type of Cinnamomum camphora oil, which has been sometimes passed off as oil of E. globulus. The British Pharmacopoeia 2002 monograph for E. globulus presents a superior GC trace to the one in this book, which would allow this distinction to be made. So perhaps BP standards are working at a better level than appreciated by the author….
p127. Components of Eucalyptus citriodora are stated to contain: “citronellal 56%, citronellol 8% (no isomer stated) 1,8 cineol 2%, a-terpinyl acetate (2%), citronellyl acetate 11.5%, and 5.5% citronellic acid (!)”.
Comment: The peculiar oil above described in the book would even fail the ISO 3044 specification (ISO is described in the book as largely set up for the food and cosmetics industry!). The analysis of the oil is presented in glib form, as if there weren’t any associated problems. Lets have a look at the real situation. Citronellal in the oil of Eucalyptus citriodora occurs as racemic b- citronellal, whereas purified synthetic citronellal is a mixture of racemic a- & b- forms. Citronellal also polymerises readily, causing problems for analysts determining the absolute citronellal content of oils. Even fresh standards of citronellal show polymerisation effects, and some analysts have resorted to preparing their own internal standards from decomposing bisulphite complexes. Additionally, citronellal in all its forms, cyclises readily, especially under the slightly acidic conditions of steam distillation, or on prolonged storage where the acid number of the oil might be high – as here, with 5.5% citronellic acid. Cyclisation leads to a number of possible isopulegol isomers (four), which may interfere with the GC determination of citronellal. Some of these isomers (but not all) are identified in the published ISO 3044 methodology, & the guidance GC traces.
Overall a weird inclusion – wouldn’t it be better to write about a typical E. citriodora oil?
p132-133 Lavender/Lavandin GC traces. Again the traces in the David Williams book appear superior.
p142 “Rose oil….may be diluted by the addition of chemicals such as PEA, DEP, citronellol and geraniol and with fractions of other oils such as geranium. The absolute may be adulterated with synthetic fractions of oils such as Peru and clove bud absolute.”
Comment. I’ve been analysing oils on an almost daily basis for 30 years and I’ve never seen any adulterated in the way described above. For a start it’s completely unlikely to fool anybody, so they wouldn’t sell any product. However I have seen rose oils adulterated by clever (chirally correct) reconstitutions… but these don’t seem to be mentioned…
p143 Rosemary oil: after describing the principle geographic types, and bearing in mind that a large volume of rosemary oils are adulterated, especially the Spanish origin featured in the GC trace, we are still not told how to ascertain if the oil is authentic. A GC trace is pretty useless without an expert interpretation…
p156 Double entry for Germacrene D in the Ylang Complete analysis. Perhaps the first entry should have read: Germacrene B 2.36%??
p180. “Peppermint and cinnamon essential oils are particularly likely to persist on the skin of the fingers for a prolonged time”. Trying to convert this into a semi-scientific statement, we could say that the evaporation rate from the dermis might show a first order rate kinetics for most oils and second order kinetics for aldehyde-containing oils. This is possibly because aldehydes form addition compounds with reactive sites on dermal proteins. In addition to this, an explanation might lie with the fact some essential oils show a greater odour intensity than others, they differ in evaporative profiles, they have different perception thresholds…
However why would an aromatherapist be using cinnamon essential oil? Both the leaf and bark oils of cinnamon present potential toxicological problems…
p185. “The LD50 values are known for most essential oils, but they represent a measure of acute oral value.”
Comment: Toxicologists have been telling us for years that theLD50 value alone is insufficient for comparisons of relative toxicity.
p185 “The amounts (presumably of essential oil?) entering the body in aromatherapy or massage are very small so they are considered to be safe”.
Comment: …Considered to be safe by whom exactly? I know of know no studies involving hardworking aromatherapists who are continually exposed to essential oils in the course of their work, and who have been monitored for oil constituent levels in body tissue, or yet have been checked for effects of sub chronic toxicity etc. etc. On the one hand, subchronic inhalation of complex fragrance mixtures has been found not constitute a risk to rodents even when inhaled under repeated and exaggerated exposure levels (Fukayama MY et al. 1999). But on the other hand are their things we haven’t evaluated - Professor Arnold Shecter for example, comments on the extreme carcinogenicity of methyl eugenol (which is present in several essential oils – see www.users.globalnet.co.uk/~nodice/ new/magazine/cropwatch3/cropwatch3.htm).
So, we see glib statements from the author on safety without any real discussion of the issues. Not good enough for a primer for students!
p185 “It has been estimated that an oral dose of an essential oil will have 10 times greater concentration than from a massage”.
Comment: Again the wording is so sloppy, it is difficult to understand the meaning intended. Who estimated these facts, and is the author of the data talking of oil concentrations in plasma, or whole body dosing, or just what exactly? And why should this be relevant? Aromatherapists may inhale essential oil vapours, and absorb oils via the hands for eight or ten hours a day 5 or six days a week. This is a different prospect from imbibing a single oral dose as far as I can see, since the toxicity of some oil components will vary according to mode of administration e.g. 1,8-cineole is orally quite toxic, but quite less so when inhaled.
p188 “…lavender and tea tree can be used with caution directly to the skin”.
Comment: Hopefully readers will realise that professional aromatherapists do not actually do this under any circumstances whatsoever, and the code of practice of several leading aromatherapy professional organisations expressly forbids it. And you shouldn’t do it either…
p198 “A small number of essential oils have been shown to cause sensitisation…. and surprisingly, ylang ylang”
Comment: Well not such a surprise, really. For example Nakayama (1998) reports on a five year project in Japan to find common fragrance sensitisers in cosmetics and to replace them with safe alternative fragrances. Ylang ylang oil was identified as a common cosmetic sensitiser in this exercise. Hypoallergenic ylang-ylang oil has subsequently been made commercially available, but does not appear to have totally eliminated these contact sensitivity problems.
Conclusion.
Although there are good spots, a sort of malaise seems to hang over whole sections of the book. For me, the book doesn’t make the subject particularly come alive…. David Willam’s book by contrast seems to be far more readable, and I should say, far more fired-up!
Because of the poor level of chemistry, lack of biochemistry and the quite basic level of chemical analysis presented, not forgetting the quite dubious safety content in places, I hesitate to recommend this book to aromatherapists.
References:
Fukayama MY et al. (1999) "Subchronic inhalation studies of complex fragrance mixtures in rats and hamsters" Toxicol Letters20, 111(1-2) 175-87.
Nakayama H. (1998) “Fragrance hypersensitivity and its Control” in P.J. Frosch, J.D. Johansen & I.R. White (eds) Fragrances – Beneficial and Adverse Effects pub. Springer-Verlag 1998.
Steltenkamp RJ et al. (1980) “Citral: a survey of consumer patch-test sensitisation” Food & Chemical Toxicology18, 413-417.
Addenda MORE BOLLOCKS
I picked up the book for another 5 minutes, and found some more….
p65 “Ketones are not very common in the majority of essential oils”.
Comment: Is this really true that ketones are uncommon in essential oils? Here is a brief round up: turmeric oil consists of 65% sesquiterpene ketones and rue oil is well for its aliphatic ketone content (2-nonanone occurring up to 90% in Algerian rue oil). European varieties of calamus oil have sesquiterpene ketones (shyobunone, acorenone etc.) as major components. Thujones occur in mugwort, wormwood, tansy, Dalmatian sage, armoise, and cedarleaf oils. Pulegone and substituted pulegones occur in some Mentha, Nepeta and Barosma species. Pinocamphones occur in hyssop and cistus oils. Pinocarvone occurs in Roman chamomile oil, hyssop oil and Cedronella canariensis (sometimes mistakenly called Balm of Gilead). Fenchone occurs in fennel, & cedarleaf oils as well as wormwood & tansy. Camphor isomers occur in oils from the camphor tree Cinnamomum camphora and in sassafras oil, spike lavender & other lavender oils, coriander, ho, & rosemary oils, some basil oils (especially hoary basil), and various sage & thyme oils, as well as mugwort and tansy oils. Methyl amyl ketone occurs in clove and lavender oil, Tagetones & Dihydrotagetones make up a major portion of tagetes oils, and tagetone & ocimenone are important in the odour of labdanum resinoid. Specific Carvone isomers occur in Mentha oils (the laevo- isomer notably spearmint & Bulgarian rose oils; the dextro- isomer in caraway & dillweed oil). Dihydrocarvone isomers occurs in dill herb oil. Menthone and Isomenthone isomers occur in oils from Pelargonium and Mentha species (certain cornmint species to 80% menthone), as well as Calamint and Buchu leaf oils. Nookatone occurs in grapefruit oil, many regarding it as the character compound. Artemisia ketone occurs in wormwood, lanyana, and santolina oils (the latter to 45%). Methyl heptenone occurs in many oils such as Litsea cubeba, lemongrass, citronella, geranium, rosewood, and verbena. Methyl vinyl ketone and 1-octen-3-one and their derivatives are important in lavender oil. Damascones and damascenones have been found to be crucial components of rose headspace odour….and then we have other ketones such as ionones (rose, boronia and the almost unobtainable violet flower oil), irones (orris oil), verbenone (rosemary oil, Spanish verbena & eucalyptus oils), piperitone (Eucalyptus dives Schauer, peppermint & perilla oils), piperitenone (catnip & horsemint oils), acetophenone (labdanum oil), cryptone (Eucalyptus polybractea, cumin seed oil, dwarf pine needle), cis-jamsone (jasmin absolute) etc….. Amongst the sesquiterpene ketones, germacrone is the main odourant of Zadravetz oil, and both geramacrone and beta-elemone contribute to the odour profile of myrrh oils, whilst alpha- and beta-vetivones are well known components of vetiver oil. Diketones such as 3,5-dimethyloctane-4,6-dione) are present in the flagship healing oil of aromatherapy: Helichrysum oil from Corsica. High levels of the triketones flavescone, isoleptospermone, and leptosepermone are found in Manuka oil, also used in aromatherapy, which have been advised as offering a high level of anti-microbial activity against gram positive micro-organisms.
Basically then, this is an another sweeping statement, with no basis in fact.
p74 “Oxides…usually made from an alcohol, and are named after the alcohol with the termination oxide e.g. linalool oxide”
Comment. Incorrect. Even the examples given show this can’t be true – alpha-pinene oxide and limonene oxides are both monoterpene hydrocarbons and can be produced by epoxidation (of a double carbon-carbon bond). Caryophyllene oxide is a sesquiterpene oxide again from a parent hydrocarbon etc.
p76 “Sulfur-containing compounds: these are not derived from terpenes.”
Comment: Totally wrong of course. Here are some terpenes containing sulphur in essential oils:
Mint sulphide occurs in Scotch spearmint & rose oil (amongst others)
p-Menth-1-en-8-thiol is a character compound of grapefruit oil
8-Mercapto-p-menthan-3-one occurs in buchu oil.
Thiocineole occurs in Eucalyptus absolute
1,2-Epithiohumulene, 4,5-epithiohumulene, 4,5-epithiocaryophyllene are trace constituents of rose oil.
Caryophyllene-6,7-episulphide, humulene-9,10 and –6,7-episulphides are all found in hop oil …and so on.
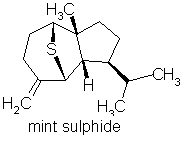
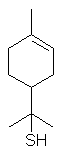
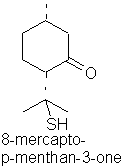 p-menth-1-en-8-thiol
p-menth-1-en-8-thiol
p76 “…phenyl ethyl ether (is) found in pandanus essential oil.”
Comment: Incorrect. Phenyl ethyl methyl ether is found in pandanus essential oil from Pandanus odoratissimus L.
FURTHER ADDITIONS 22.08.04
p55 “Thyme at very bottom of mountain: oil contains significant amounts of phenol.”
Comment: Phenol, quite a dangerous material to the skin, is not found in essential oils.
p65 “Carvone is found in gripe water and seems to be harmless.”
Comment: (+)-carvone is more toxic than (-) carvone and interestingly was formerly classified as an S1 poison. If you do an internet search for MSDS for (+)-carvone you will see data for the LD50 orl-rat is 3.7 mg kg suggesting that the pure substance is in fact extremely orally toxic to rats, at least.
p68 “Veleranone.”
Comment: Presumably a typo – should be valeranone in valerian oil.
p68 “Many esters present in essential oils are formed as reaction products during the distillation process in extraction”
Comment: Absolutely priceless! :-) :-) Made my day! :-) :-)
Although the biogenic pathways of compounds found in essential oils are not covered in this book, I think if the author took a course on biochemistry she would find that esters are not produced in this manner. On the contrary the lowish pH at which most steam distillations occur 4-5 or lower actually tends to promote loss of existing esters by hydrolysis. Further some esters such as methyl salicylate in Wintergreen oil are bound to glycosides in the plant material, and have be treated to be freed of their sugar moieties before they can be distilled and isolated.
p80 “Steam distillation is quick..”
Comment:Not always so: Ylang ylang may take 24 hours or more; sandalwood oil 2-3 days to get the crude, 2-3 days to redistill; most wood oils take 1-3 days after soaking the chips or sawdust for 24 hours.
p82 “An absolute may not have all the volatile oils from a plant, it will only have those that are alcohol soluble.”
Comment: Surely all the volatile oils from the plant are alcohol soluble? Perhaps the author meant to say that some classes of compounds from the plant (waxes, flavanoids) will not be alcohol soluble?
p86 Lavandin essential oil is used as a source of linalol for the perfume industry.
Comment: Nowadays overtaken by linalol ex ho oils, which are much cheaper, sometimes even cheaper than synthetic linalol itself.


